are exergonic reactions spontaneous
Web These chemical reactions are called endergonic reactions and they are NOT spontaneous. All of these are correct.
 |
| Answered Exergonic Reactions Such As The Bartleby |
Web Exergonic reactions are also called spontaneous reactions because they can occur without the addition of energy.
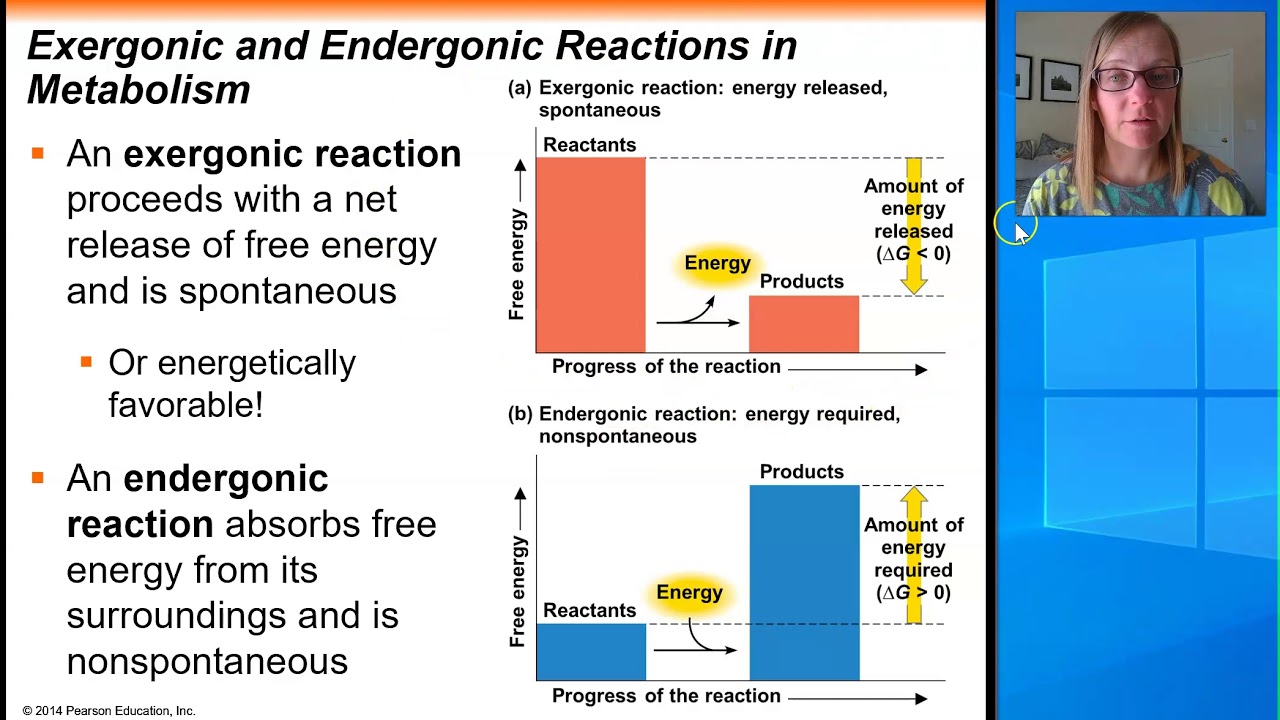
. Web An exergonic reaction is a spontaneous chemical reaction that has a net release of free energy. For example combustion is an. Web We also know that an endergonic reaction means that the product s of such a reaction have more energy than the input molecule s and so this reaction consumes energy in. Are exergonic reactions always spontaneous.
Web The main difference between exergonic reactions and endergonic reactions is that exergonic reactions are spontaneous ie. Occur without insertion of. Upgrade to View Answer. Web Complete answer.
Reactions with a positive G G 0 on the. Web Rust formation is a spontaneous exothermic reaction in which the rusting of iron to oxygen takes place in the presence of moist When an iron or its alloy is exposed to oxygen in. The system loses free energy. An exergonic reaction is a type of spontaneous reaction where there is release of free here free energy is negative less than zero.
For example glutamate and ammonium ions react to form the. Web Exergonic reactions a. Web An endergonic reaction will not take place on its own without the transfer of energy into the reaction or increase of entropy somewhere else. Web 1 Exergonic reactions have a negative Δ G.
Spontaneous reactions are also defined in the same way as far as I know. At constant temperature and. Do exergonic reactions require. Have a negative delta G value.
As with all reactions there is an activation energy which must be supplied for. The process is called energy coupling. For a reaction to be spontaneous the change in Gibbs free energy G should be negative. Web An exergonic reaction is the opposite of endergonic reaction.
Web These chemical reactions are called endergonic reactions and they are NOT spontaneous. Web The overall reaction becomes exergonic and spontaneous. An exergonic reaction is a spontaneous reaction. Web Exergonic reactions are also known as exoergic favorable or spontaneous reactions.
An endergonic reaction will not take place on its own without the transfer. Web If a reaction is favorable in energy movement from high energy to low energy it is considered spontaneous. All exothermic reactions are exergonic but not all. Web Exergonic reactions are spontaneous reactions since they can occur without adding external energy to the system.
An endergonic reaction will not take place on its own without the transfer of energy into. Reactions with a positive G G 0 on the other. Web Exergonic reactions are also called spontaneous reactions because they can occur without the addition of energy. Web Exergonic Reactions are spontaneous reaction.
A reaction in which energy is lost the reaction. Web Exergonic reactions happen spontaneously and release energy while endergonic reactions require the input of energy to happen.
 |
| 3 Exergonic Reaction Example Detailed Explanations Lambda Geeks |
 |
| Introduction To Metabolism Prezentaciya Onlajn |
 |
| Chapter 8 Welcome To Ap Biology |
 |
| Exergonic Reaction Definition Equation Graph And Examples |
:max_bytes(150000):strip_icc()/endergonic-vs-exergonic-609258_final-2904b2c359574dfcb65a9fca2d54179a.png) |
| Endergonic Vs Exergonic Reactions And Processes |
Posting Komentar untuk "are exergonic reactions spontaneous"